Immunofluorescence Tomography: High-resolution 3-D reconstruction by serial-sectioning of methacrylate embedded tissues and alignment of 2-D immunofluorescence images
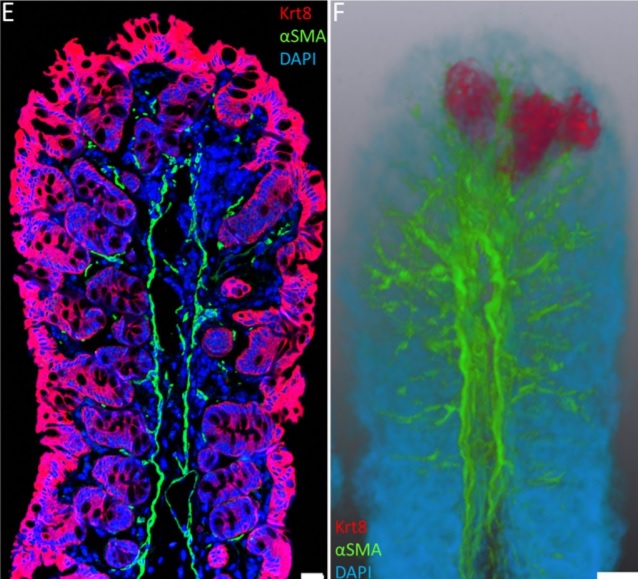
Immunofluorescence tomography is a high-resolution 3-D reconstruction method based on methacrylate embedding and serial-sectioning, where 2-D images of immuno-stained serial-sections are computationally aligned into image stacks, and the 3-D volume rendered. Butyl-Methyl Methacrylate (BMMA) plastic was adopted as it preserves excellent tissue morphology and can be de-plasticized easily using an organic solvent, which enables immuno-staining of serial-sections without antibody penetration issues over millimeters of 3-D reconstructed tissue (Z-depth). High axial Z-resolution over a large volume was achieved by cutting serial-sections at 2 µm thickness. Stained sections were imaged by multiple modalities, including immunofluorescence, electron microscopy and second harmonic generation (SHG), and there are advantages over confocal microscopy as the tissue does not need to be cleared, while antibody penetration or light scattering issues are minimized. The plastic serial-sections can be re-probed, without a loss in tissue structure, using low pH glycine hydrochloride antibody elution. It is a cost-effective approach as the microscopes needed are significantly cheaper than confocal microscopes and sections can be kept indefinitely. Therefore, immunofluorescence tomography is a powerful new tool to quantify sub-populations of cells in high-resolution 3-D using antibody fluorescence. This article describes the immunofluorescence tomography method for 3-D reconstruction of epithelial tissues such as mammary gland, cornea and the hair follicle.