In Situ Synthesized La0.6Sr0.4Co0.2Fe0.8O3−δ–Gd0.1Ce0.9O1.95 Nanocomposite Cathodes via a Modified Sol–Gel Process for Intermediate Temperature Solid Oxide Fuel Cells
Composite cathodes comprising nanoscale
powders are expected to impart with high specific surface
area and triple phase boundary (TPB) density, which will lead
to better performance.
However, uniformly mixing nanosized heterophase powders remains a challenge due to their high surface energy and thus ease with which they agglomerate into their individual phases during the mixing and sintering
processes. In this study, we successfully synthesized La0.6Sr0.4Co0.2Fe0.8O3−δ (LSCF)−Gd0.1Ce0.9O1.95 (GDC) composite
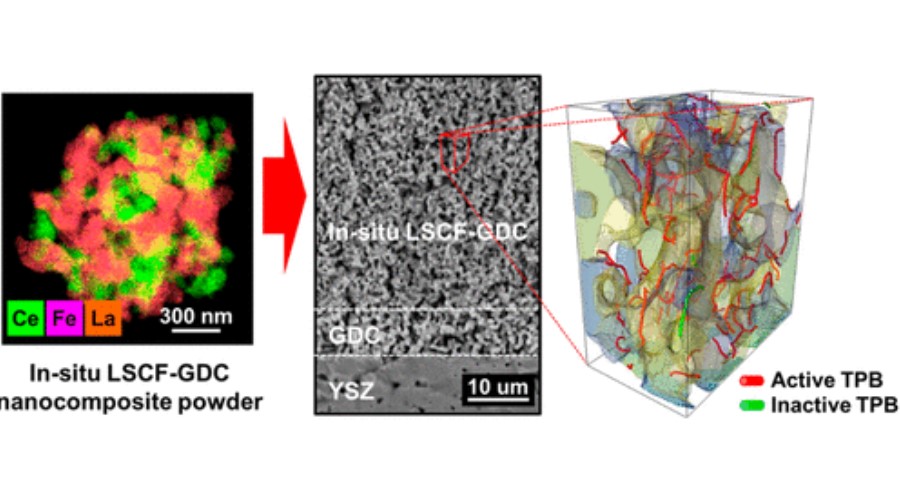
cathode nanoscale powders via an in situ sol−gel process. High-angle annular dark field scanning transmission electron microscopy analysis of in situ prepared LSCF−GDC composite powders revealed that both the LSCF and GDC phases were uniformly distributed with a particle size of ∼90 nm
without cation intermixing. The in situ LSCF−GDC cathode sintered on a GDC electrolyte showed a low polarization resistance of 0.044 Ω cm2 at 750 °C. The active TPB density and the specific two phase (LSCF/pore) boundary area of the in situ LSCF−GDC cathode were quantified via a 3D reconstruction technique, resulting in 12.7 μm−2 and 2.9 μm−1, respectively. These valuesare significantly higher as compared to reported values of other LSCF−GDC cathodes, demonstrating highly well-distributed LSCF and GDC at the nanoscale. A solid oxide fuel cell employing the in situ LSCF−GDC cathode yielded excellent power output of ∼1.2 W cm−2 at 750 °C and high stability up to 500 h.