New Method for the Deposition of Nickel Oxide in Porous Scaffolds for Electrodes in Solid Oxide Fuel Cells and Electrolyzers
A simple chemical bath deposition is used to coat a complex porous ceramic scaffold with a conformal Ni layer.
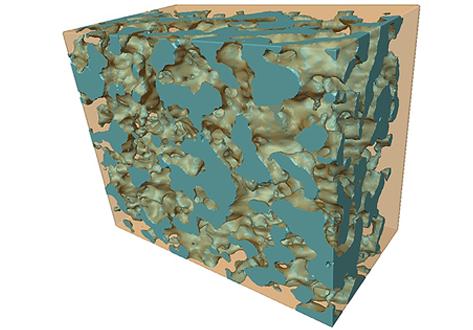
The resulting composite is used as a solid oxide fuel cell electrode, and its electrochemical response is measured in humidified hydrogen. X‐ray tomography is used to determine the microstructural characteristics of the uncoated and Ni‐coated porous structure, which include the surface area to total volume, the radial pore size, and the size of the necks between the pores.
The use of metal infiltration into a ceramic scaffold to fabricate electrodes for solid oxide fuel cells and electrolyzers (SOFC/SOEC) has been demonstrated successfully in terms of electrochemical performance. The concept is to first fabricate a ceramic scaffold for the electrolyte and then deposit NiO by infiltration. During infiltration, the pores are saturated with a metal nitrate solution and then treated thermally at 500–700 °C to decompose the solution into the metal oxide. The incorporation of two electrode components, for example, BaCe0.5Zr0.3Y0.16Ni0.04O3−δ (BCZYZ) and Ni, at different processing stages means that the microstructure of the electrode can be controlled independently for each component. The electrode is expected to be more tolerant to redox‐cycling as the volume changes experienced by Ni can be accommodated by the porous structure. Infiltration, also known as impregnation, yields excellent results for Ni/Gd‐doped ceria and for Ni/BCZYZ in terms of Ni distribution and low polarization resistance. Nonetheless, infiltration is a time‐ and energyconsuming technique and its scale‐up is problematic. Furthermore, the instability of the infiltrated nanostructure needs to be addressed as initial work indicates that the infiltrated material can degrade rapidly depending upon the number of infiltration cycles.