Preclinical Evaluation of 203/212Pb-Labeled Low-Molecular-Weight Compounds for Targeted Radiopharmaceutical Therapy of Prostate Cancer
Targeted radiopharmaceutical therapy (TRT) using α-particle radiation is a promising approach for treating both large and micrometastatic lesions. Researchers developed prostate-specific membrane antigen (PSMA)–targeted low-molecular-weight agents for 212Pb-based TRT of patients with prostate cancer (PC) by evaluating the matching γ-emitting surrogate.
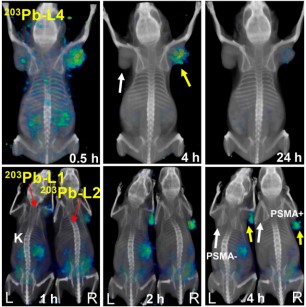
Targeted radiopharmaceutical therapy using α-particles (α-TRTs), which cause deposition of ionizing radiation of high-linear-energy transfer, is accelerating in importance for managing prostate cancer (PC). This acceleration is due in part to the unexpected survival benefit conferred by 223RaCl2 in patients with castration-resistant PC metastatic to bone. Also contributing to this acceleration has been the remarkable decrease in tumor burden demonstrated on images of patients who received 225Ac-PSMA-617, which targets prostate-specific membrane antigen (PSMA) in patients with metastatic castration-resistant PC who failed prior standard treatment. However, salivary and lacrimal gland radiotoxicity may affect the overall survival benefit by reducing quality of life. As an alternative to 225Ac (half-life, 10 d), 212Pb, which has a shorter physical half-life (10.6 h), is a promising source of α-emissions that has proved safe and effective in both preclinical models and clinical studies for several indications. 212Pb is commercially available from a 224Ra generator and has well-described radiochemistry. It is a β-emitter but serves as an in vivo nanogenerator of 212Bi (half-life, 1.01 h), which decays with an α-particle in its decay chain. 212Pb has been successfully used as a stand-alone treatment and in combination with chemotherapy using peptides and monoclonal antibodies as targeting vectors. Although PSMA-based TRT using low-molecular-weight agents and monoclonal antibodies is expanding in management of metastatic castration-resistant PC, to date this has primarily used agents that deliver β-emitting payloads. Few preclinical studies describe detailed evaluation of α-TRT.